Background: Current treatment of unfit acute myeloid leukemia (AML) patients include hypomethylating agents (HMA) with or without venetoclax, low-dose cytarabine (LDAC), and supportive care only. Recently, the combination of azacytidine with ivosidenib, an IDH1 inhibitor, has showed a significant improvement in survival in unfit patients with IDH1 mutated (IDH1mut) AML compared to azacytidine plus placebo. Real world studies analyzing outcomes of IDH1mut AML patients are scarce.
Aims: This study aims to assess retrospectively the characteristics, therapeutic approaches, and outcomes of unfit patients with AML in an unselected population reported to the multicentric PETHEMA registry according to IDH mutational status. We present here a first interim analysis, as the study is aimed to enroll 3500 patients.
Methods: AML unfit patients reported to PETHEMA registry between January 2015 and June 2022 were included in the study, regardless of their therapeutic approach. IDH1 mutational status was analyzed with next generation sequencing (NGS) and polymerase chain reaction (PCR) technics performed in central labs from PETHEMA group, as well as locally as per standard practice. All clinical records were reviewed from diagnosis to death/last follow-up and data were analyzed with R statistical software.
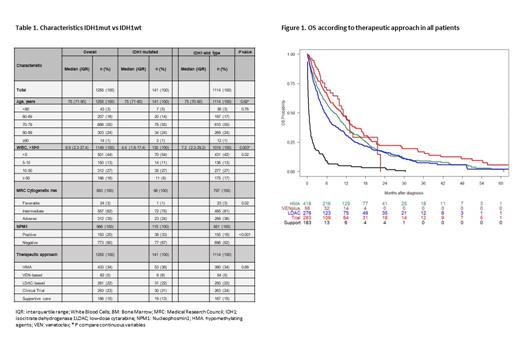
Results: From 9398 patients reported to the PETHEMA registry between 2015 and 2022, 4533 patients had information on IDH1 mutational status. Of them, 2096 patients were unfit for intensive chemotherapy, and therapeutic approach was available in 1255 patients who were evaluable for this interim report. Median age was 75 years, 716 (57%) were male, 312 (35%) had adverse cytogenetic-risk. Overall, 141 (11%) had IDH1mut [106 (12.4%) by NGS and 77 (9.9%) by PCR only] and 1114 (89%) had no IDH1 mutation (IDH1wt). There were differences between IDH1mut vs IDH1wt cohorts regarding white blood cells (WBC) count (P=0.003), platelets count (P=0.002), bone marrow blasts percentage (P<0.001), MRC cytogenetic risk (P=0.02) and NPM1 mutational status (P<0.001). More detailed information is shown in Table 1.
Regarding treatments, 176 (15%) patients received only best supportive care (BSC), 293 (23%) were included in non-intensive clinical trials, 433 (34%) received HMAs, 281 (22%) LDAC schedules and 62 (5%) venetoclax plus HMA or LDAC (VEN). From 818 patients with available response, 261 (32%) achieved complete remission (CR) or CR with incomplete recovery (CRi), 105 (13%) partial remission, 305 (37%) were resistant and 147 (18%) died before being assessed. No statistical differences were observed between IDH1mut and IDH1wt groups (P=0.18).
Median overall survival (OS) was 5.9 months (CI95, 5.3-6.7), with no differences in IDH1mut and IDH1wt cohorts [median 7.7 months (CI95, 5.9-11.2) vs 5.6 (CI95, 5-6.4)], with a 2y-OS of 15% (9.7-24.5) vs 15% (12.9-18.2), respectively (P=0.24). Median OS was 7.9 (CI95, 6.8-9.3) in patients receiving active treatment. Patients receiving VEN schedules had a median OS of 11.1 months (CI95, 9.4-14.1) vs 10.5 (CI95, 8.2-13.0) in patients included in clinical trials vs 8.3 (CI95, 6.7-10.0) in those receiving HMA vs 5.5 (CI95, 4.5-6.8) treated with LDAC-based schemes vs 0.4 (CI95, 0.3-0.7) in BSC patients (P<0.001). Figure 1 shows OS according to therapeutic approach in all patients.
According to the therapeutic approach, IDH1mut patients included in clinical trials had a median OS of 16.8 (CI95, 6.0-NA) vs 10.6 months (CI95, 9.9-NA) in patients receiving VEN schedules vs 9.0 (CI95, 6.5-14.5) in those receiving HMA vs 7.0 (CI95, 5.5-12.7) with LDAC-based vs 0.6 (CI95, 0.2-3.7) in BSC patients (P<0.001).
Conclusion: We show a prevalence of 11% of IDH1 mutation among patients considered unfit to receive intensive chemotherapy. Although there were differences between IHD1mut and IDH1wt subgroups in some variables with demonstrated prognostic impact, no significant differences were observed in response to treatment or OS.
This study was partially supported by Servier.
Disclosures
Martinez-Cuadron:Pfizer: Other: Travel, Accommodations; Otsuka: Consultancy, Other: Travel, Accommodations; Astellas: Consultancy, Speakers Bureau. Bergua Burgues:Fundesalud. Grants of Europena funds.Daychii: Research Funding; Daychii: Consultancy; Hospital San Pedro de Alcántara. Servicio de Hematologia. Cáceres. SPAIN: Current Employment. Tormo:BMS: Honoraria; Astellas: Honoraria; Pfizer: Honoraria; MSD: Honoraria; AbbVie: Honoraria. Lavilla:Sanofi, Janssen, Incyte, SOBI; Belgene: Consultancy, Honoraria. Bernal Del Castillo:Otsuka: Consultancy; Jazz: Consultancy; AbbVie: Consultancy. Ayala:BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Speakers Bureau. Montesinos:Ryvu: Consultancy; Astellas: Consultancy, Speakers Bureau; Novartis: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; GILEAD: Consultancy; OTSUKA: Consultancy; BEIGENE: Consultancy; INCYTE: Consultancy; NERVIANO: Consultancy; Celgene: Consultancy; Janssen: Speakers Bureau; Kura oncology: Consultancy; Menarini-Stemline: Consultancy, Research Funding; Jazz pharma: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Research Funding, Speakers Bureau; BMS: Consultancy, Other, Research Funding; Daiichi Sankyo: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal